Jul 01, 2016
Water does work. It physically wears down towering mountains to stumpy hills through erosion. The movement of water provides a constant flow that feeds the vast networks of lakes, rivers, and groundwater that we depend on. But water controls many other aspects of our lives that we cannot see, particularly the chemistry of our environment. One of the unsung chemical actions of water is the process of leaching. Although its name might bring about thoughts of leeches and other creepy-crawlies, it is something very different. So, what is leaching?
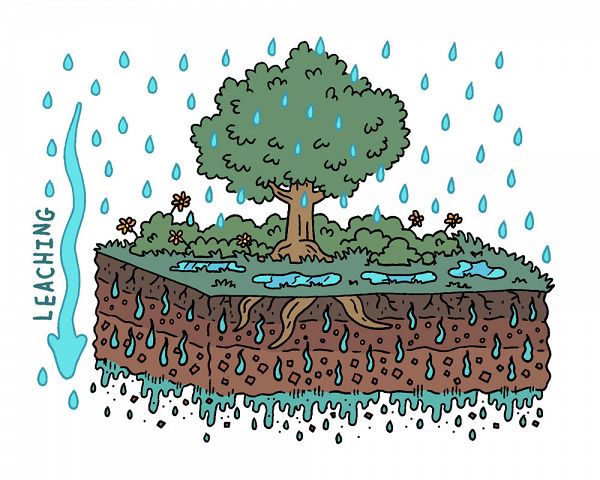
Image 1. Water leaching through the Critical Zone. Rain falls on a cross section of a tree atop a soil profile. Rain falls on vegetation, pools on the ground, and leaches into and down through the soil profile.
Leaching is not to be confused with the use of leeches as medicine. The word “leaching” is believed to have been derived from either late Middle English “leche” or Old English “leccan” meaning to moisten and to allow leaking. Currently, leaching primarily describes the process of water carrying soluble substances or small particles through soil or rock. Although this process seems trivial, leaching is one of the key processes of the Critical Zone, controlling the rate and direction in which compounds move.
Leaching is actually two important actions occurring simultaneously: (1) chemical interactions with surfaces and (2) physical movement of water. As the water passes through the rock and soil, it interacts with the surfaces of the materials. Compounds on the surface of minerals can be become dissolved. In addition, the physical movement of water can dislodge and move particles. Leaching can transport chemical compounds like dissolved substances or larger materials such as decomposing plant materials, fine rock fragments, and microbes throughout the Critical Zone.
In agricultural ecosystems, leaching is an important balance between preventing salt accumulation and removing nutrients from soil. In dry soils of semi-arid regions salts can accumulate in the top horizons of the soil. The Food and Agriculture Organization of the United States estimated 45 million hectares of the 230 million hectares of irrigated croplands are salt-affected. These soils suffer from the accumulation of salts due to limited leaching. Without proper amounts of water to leach these salts (known as the leaching fraction) from the upper soil horizons, the growth of the plants can be slightly to severely impacted. The impact depends on the salt tolerance of the plant and the type of salts accumulating in the soil.
On the other hand, excessive leaching can flush nutrients from the soil, especially nitrate and phosphate. According to Extension Specialists in Nutrient Management at the University of Minnesota, excessive leaching of nitrate has the potential to affect groundwater and lakes from tile drainage. The movement of nitrate and other compounds in agricultural drainages is an important question being studied at Intensively Managed Landscapes (IML) CZO. Understanding water in agriculture or ‘Hydrocomplexity’ is part of the ‘Blue Revolution’, as described by Dr. Praveen Kumar (Kumar, 2015).
Leaching can also affect weathered rocks and even bedrock. Water is able to fit into small spaces between the mineral grains and inside tiny fractures in the rock. As additional water moves into the rock, it can cause the leaching of elements from rocks in two ways: dissolution and oxidation. Subsurface movement of water can cause dissolution of sedimentary rocks, especially calcium carbonate rocks like limestone. This can lead to hazards such as sinkholes and erosion.
When water moves into rocks, it commonly carries oxygen with it. The introduction of oxygen to rocks formed under low oxygen conditions causes a cascading chemical reaction allowing for once immobile metals to become rapidly mobilized. This oxidation caused by leaching is one of the main drivers of acid mine drainage! Once leaching of iron and other previously unoxidized metals begins, it is difficult to reverse the resulting acid mine drainage (Check out this article on acid mine drainge by the BBC).
Leaching may also occur high above soil, regolith, and rock, in the tree tops. As rainfall lands upon the leaves, the water interacts with the leaf surface and may accumulate dissolved ions from the leaf. This process of rainfall removing dissolved ions from leaves is commonly referred to as foliar leaching. In a study of temperate deciduous trees at the Christina River Basin by Van Stan et al (2012), rainfall significantly leached potassium, calcium, and magnesium from leaves. However, the authors found this to be dependent on the season and intensity of the rainfall.
These are only a few examples of leaching and many more exist!
Have any questions swirling in your noodle about the rock, soil, water, fauna, or flora of the Critical Zone? Send them our way at Askcriticalzone@gmail.com.
Science on!
Justin Richardson
Critical Zone Observatory Postdoctoral Fellow
References
Kumar, P. (2015): Hydrocomplexity: Addressing Water Security and Emergent Environmental Risks. Water Resources Research, 51: pp12. DOI: 10.1002/2015WR017342
Van Stan, J.T., D.F. Levia, S.P. Inamdar, M. Lepori-Bui, & M.J. Mitchell (2012): The effects of phenoseason and storm characteristics on throughfall solute washoff and
Image 1. Water leaching through the Critical Zone. Rain falls on a cross section of a tree atop a soil profile. Rain falls on vegetation, pools on the ground, and leaches into and down through the soil profile.

Justin B. Richardson
CZO INVESTIGATOR, STAFF. National Office outreach officer, Former CZO Post-Doctoral Fellow. Specialty: Soil biogeochemistry of plant-essential and toxic metals.
Hydrology Soil Science / Pedology EDUCATION/OUTREACH General Public K-12 Education
COMMENT ON "Adventures in the Critical Zone"
All comments are moderated. If you want to comment without logging in, select either the "Start/Join the discussion" box or a "Reply" link, then "Name", and finally, "I'd rather post as a guest" checkbox.
ABOUT THIS BLOG
Justin Richardson and his guests answer questions about the Critical Zone, synthesize CZ research, and meet folks working at the CZ observatories
General Disclaimer: Any opinions, findings, conclusions or recommendations presented in the above blog post are only those of the blog author and do not necessarily reflect the views of the U.S. CZO National Program or the National Science Foundation. For official information about NSF, visit www.nsf.gov.
Explore Further