Weathering & Soils Research Group
We seek to advance our understanding of soil development as a function of climate, of Fe tranformations during weathering and soil developmemt, of mineral reactions in and below the soil, of nanoscale feature evolution in shales during weathering, and of 1D, 2D and 3D weathering rates.
Image: Group photo from December 2011
This group is tagged with:
-
Sources and cycling of Mn: Located in the heart of a historic iron producing region, the Susquehanna Shale Hills CZO provides a model system to investigate anthropogenic metal inputs to soils. Detailed geochemical studies have shown that excess manganese (Mn) contaminates the soils at SSHO due to past atmospheric deposition (Herndon et al., 2011).
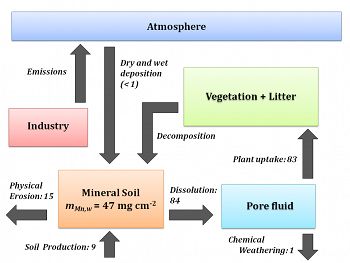
This Mn, emitted as a byproduct of iron and steel production, coal combustion, and other industries, remains in the soil as the legacy of Huntingdon County's industrial past.Figure 1. A box model shows mass reservoirs of Mn (boxes) and the Mn fluxes that impact SSHO (arrows; values in μg cm-2 y-1). Emissions from anthropogenic activity have resulted in a net gain of Mn in SSHO soils (mMn, w = 47 mg cm-2). Rates of Mn uptake into trees (83 μg cm-2 y-1) currently outpace removal through chemical weathering (1 μg cm-2 y-1), indicating that vegetation may retain Mn within the catchment.
We are currently evaluating the role of vegetation in retaining Mn within the watershed (Figure 1). Low concentrations of Mn are found in soil pore fluids, indicating that losses to chemical weathering are small. In contrast, high concentrations of Mn are found in tree leaves, suggesting that Mn is being rapidly taken up from soil by the trees. In this way, vegetation acts as a “capacitor” that stores Mn and releases it slowly to the environment over time. We are using X-ray absorption spectroscopy (XAS) to characterize Mn in soils and vegetation. Preliminary results show that Mn in foliar tissue is reduced and complexed with organic compounds (Figure 2), while Mn in soils is present as a tri-/tetravalent oxide, indicating that Mn is oxidized and immobilized as tree leaves fall to the soil and are decomposed.
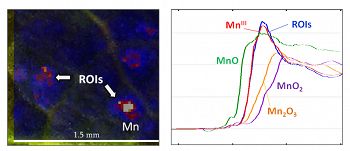
Additionally, we are coupling our study of Mn at SSHO with analysis of global Mn contamination. Data gathered from multiple geochemical databases show that Mn addition is common in soils, but patchy in distribution. Air and water data indicate that air concentrations of Mn peaked in the 1960s before declining by an order of magnitude, while riverine outputs of Mn peaked ~1980 before also declining.Figure 2. X-ray fluorescence spectroscopy (XRF) and X-ray absorption spectroscopy (XAS) are used to characterize the location and speciation of manganese within leaf biomass. Left: Red oak leaves examined with XRF exhibit discrete areas of high Mn, ROIs (regions of interest), as shown in red and white. Right: XAS was used to analyze Mn hot spots within foliar tissue. Mn spectra obtained from these ROIs (shown in blue) are most consistent with trivalent, organically-complexed Mn. Standards for the oxides MnO, Mn2O3, and MnO2 are also shown.
This research is spearheaded by Elizabeth Herndon. [Herndon, E.M., Jin, L., and Brantley, S.L. (2011) Soils reveal widespread manganese enrichment from industrial sources, Environmental Science & Technology 45(1), 241-247.]
Iron Isotopes: This research involves understanding how Fe is transformed and cycled as a result of weathering and soil formation in the Shale Hills watershed. In order to investigate this question, a series of soil samples were collected from along a transect and analyzed for bulk chemistry and Fe(II) concentrations. Chemical weathering results in a moderate but incomplete loss of both total Fe and Fe(II) from soil profiles. Furthermore, mass balance calculations are used to evaluate fluxes of these two constituents from different topographic positions along the transect.
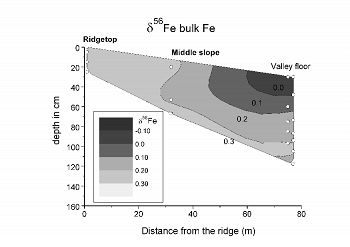
In order to better elucidate microbiological processes that may be cycling Fe within the catchment, both Fe-oxidizing and Fe-reducing bacteria were cultured. The abundance of Fe-reducing bacteria is well predicted by soil moisture level, while Fe-oxidizing bacteria are often prevalent near the soil/bedrock interface.Figure 3. A contour map showing the isotopic composition of Fe in bulk soil samples (referred to as the δ56Fe value) along the transect. Contrary to expectations from experiments involving Fe-reducing bacteria and ligand-promoted dissolution, the bulk Fe signatures appear to become lighter with increased chemical weathering toward the valley floor.
A final goal of this research involves understanding how different processes associated with Fe weathering and cycling may fractionate the stable isotope values of Fe in soils. Previous experimental work suggests that many common processes (including bacterial Fe reduction and the ligand-promoted dissolution of minerals) tend to produce Fe isotope signatures in bulk soils that are isotopically heavy relative to starting material. In contrast, although the overall extent of Fe fractionation in SSHO soils is low, we observe a very different type of trend that involves isotopic depletion with increased weathering extent toward the valley floor (Figure 3). At the moment, we are not fully sure of how to explain this trend. It may result from the preferential uptake of isotopically light Fe by vegetation over time or from the loss of isotopically enriched dissolved Fe from the watershed. This research is spearheaded by graduate student Tiffany Yesavage.
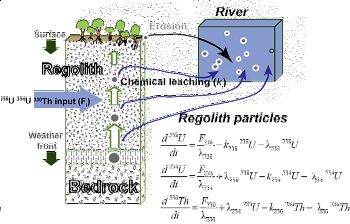
Uranium-series disequilibrium isotopes: U-series disequilibrium isotopes (238U, 234U, and 230Th) of shale rocks and soils are being analyzed at the Shale Hills catchment to derive the soil production function and estimate the residence time of regolith particles using mass balance models (Figure 4).
The activity ratios observed in the soils are explained by loss of U-series isotopes during water-rock interactions and re-precipitation of 234U and 238U downslope. Regolith production rates calculated with these isotopes decrease exponentially from 45 to 15 m/Myr (regolith residence times increase from 6.7 to 45 kyr) on the south planar transect, with increasing soil thickness from the ridge top to the valley floor. By contrast, regolith production rates from the north planar transect are ~40-52 m/Myr (regolith residence times are 8-10 kyr). The regolith profiles on the northern, sun-facing slope are thus characterized by faster regolith production rates and shorter duration of chemical weathering compared to the southern, more shaded slope. These observations are consistent with the conclusion that aspect exerts an important control on the regolith formation at the Shale Hills catchment. The aspect asymmetry induces different microclimatic conditions that affect slope stability and erosion and set the residence time for the materials available for chemical weathering. Given that the SSHO experienced a peri-glacial climate ~15 ky ago, we conclude that the hillslope retains regolith formed before that glacial period and that the hillslope is not at geomorphological steady state. This research is spearheaded by postdoctoral scholar Lin Ma (present address: Department of Geological Sciences, University of Texas at El Paso, El Paso, TX 79968). [Ma, L., Chabaux, F., Pelt, E., Blaes, E., Jin, L., and Brantley, S.L., Regolith production rates calculated with Uranium-series isotopes at Shale Hills Critical Zone Observatory, Earth and Planetary Science Letters vol 297, 211-225; Ma, L., Chabaux, F., Pelt, E., Jin, L., and Brantley, S., North versus south: Implications from U-series isotopes about regolith formation at Shale Hills, to be submitted]Figure 4. U-series disequilibrium isotopes (238U, 234U, and 230Th) of shale rocks and soils are being analyzed at the Shale Hills catchment to derive the soil production function and estimate the residence time of regolith particles using mass balance models.
Water Chemistry: An intense field study was conducted at the Susquehanna/Shale Hills Critical Zone Observatory to use water chemistry to probe the subsurface hydrogeochemical conditions and water flow dynamics. Soil pore water sampled through tension lysimeters suggest that contemporary reaction rates depend on preferential flowpaths and solute residence times, and are limited by dissolution kinetics.
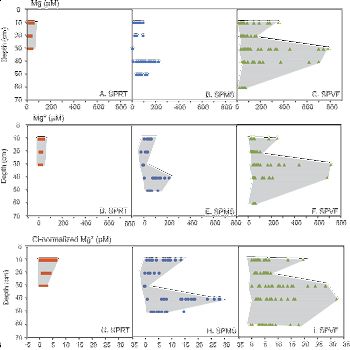
Thus weathering processes are strongly coupled with hydrological features of shale rocks and soils. The chemistry of ground- and streamwater is being analyzed in relation to that of the soil porewaters to elucidate the mineral reactions occurring below the soils and to derive elemental fluxes over the whole catchment.Figure 6. Mg2+, Mg*, and Mg* (Cl-normalized) concentrations in the pore water as a function of depth at the SPRT (A, D, G), SPMS (B, E, H) and SPVF (C, F, I) sites. Shaded areas highlighted the variations of Mg concentrations in soil waters from different depths. The Mg2+ concentration is that measured in the soil water; Mg* is Mg concentration in soil water after correcting for atmospheric input; and Mg* (Cl-normalized) is Mg concentration corrected for atmospheric input and evapotranspiration. Notice changes in scale between Mg2+/Mg* and Mg* (Cl-normalized).
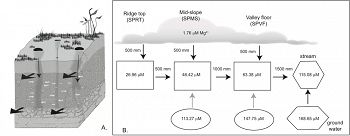
Soil water solutes are predominantly contributed by shale weathering, which is limited by clay dissolution kinetics. As such, solute concentrations are primarily controlled by the residence time of water in soil. Translocation and deposition of secondary clays created B-horizons that are more conductive to lateral flow right above or below it than vertical flow. Consistent with soil moisture monitoring data, water flows out of the steep-sloping hillslope predominantly laterally through preferred pathways that include soil horizon and soil-bedrock interfaces, and vertically via macropores. As a consequence, much higher concentrations of major cations are observed in porefluids within the B-horizon because water flows more slowly and mineral-water contact time is much longer. The amplitude of seasonal variations in O and H isotopes decreases in the order: precipitation>> soil water > stream > groundwater, suggesting water becomes progressively older as rainfall infiltrates the soil and eventually recharges to ground water. Interestingly, Mg concentrations increase with the residence time of the water due to increasing contact time with minerals (clay and ankerite). Stream water is a mixture of groundwater and shallow soil water where the relative proportions change seasonally. The discharge of the first-order stream responds to precipitation closely, documenting the rapid release of both old groundwater and relatively young soil water during storms.
Figure 7. Conceptual hydrological flow model on pedon (A) and hillslope (B) scales. In A, large wide black arrows indicate preferential flow paths, narrow black arrows indicate vertical flow through macropores formed for example by tree roots, and white curly arrows indicate solute exchange between low-flow zone and macropores via diffusion. In B, black arrows indicate water and Mg transport through rainfall and snowmelt input, advection along the hillslope through the preferential flow paths (high-flow domain, squares) and recharge to stream from vadose zone and groundwater. Grey arrows mean diffusion of Mg2+ from restrictive layers (low-flow domain, eclipse) to flow paths (high-flow domain). The Mg2+ concentrations in each reservoir are calculated by averaging those measured at corresponding sites/depths and used to determine the chlorite dissolution rates (see text for details). It is assumed that the annual precipitation is 1000 mm and half of it returns to the atmosphere via evapotranspiration. Thus the Mg concentration in water that penetrates to the soil doubled (=0.88 *2=1.76 mM).
[Jin, L., Andrews, D.M., Holmes, G.H., Duffy, C.J., Lin, H and Brantley, S.L. (2011) Opening the "Black Box": Water chemistry reveals hydrological controls on weathering in Susquehanna/Shale Hills Critical Zone Observatory (Central Pennsylvania, USA), Vadose Zone Journal 10:928-942.]
Neutron Scattering: We use neutron scattering techniques to characterize the evolution of nanoscale features (e.g., nano-porosity, surface area, surface roughness) in shales and to reveal that changes of these physical properties are coupled with chemical weathering reactions.
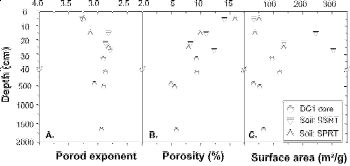
At ~ 20 m depth, dissolution is inferred to have depleted the bedrock of ankerite and all the chips investigated with neutron scattering are from above the ankerite dissolution zone. Scattering data documents that 5-6% of the total ankerite-free rock volume is comprised of isolated, intraparticle pores. At 5 m depth, an abrupt increase in porosity and surface area corresponds with onset of feldspar dissolution in the saprock and is attributed mainly to peri-glacial processes from 15 ka. At tens of centimeters below the saprock-regolith interface, the porosity and surface area increase markedly as chlorite and illite begin to dissolve. These clay reactions contribute to the transformation of saprock to regolith. Throughout the regolith, intraparticle pores in chips connect to form larger interparticle pores and scattering changes from a mass fractal at depth to a surface fractal near the land surface. Pore geometry also changes from anisotropic at depth, perhaps related to pencil cleavage created in the rock by previous tectonic activity, to isotropic at the uppermost surface as clays weather. In the most weathered regolith, kaolinite and Fe oxyhydroxides precipitate, blocking some connected pores.Figure 8. Variations of power law Porod exponent (A), porosity (B), specific surface area (C), and ferrous and total Fe contents as a function of depth (D), for shales that have been weathered to different extents. The exponent, n, indicates the type of fractal (2 < n < 3, mass fractal; 3 < n< 4, surface fractal).
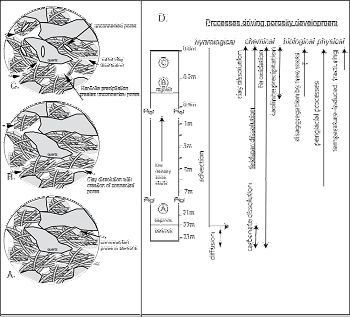
These precipitates, coupled with exposure of more quartz by clay weathering, contribute to the decreased mineral-pore interfacial area in the uppermost samples.Figure 9. A schematic diagram summarizing the production and changes in connectivity of the pores as measured by neutron scattering, and the processes that are causing changes in porosity at different depths. Initially, shales are dominated by unconnected type II pores (A). With weathering, pores become more connected by loss of clays and type III pores dominate (B). With progressive weathering, more space opens up due to clay dissolution, which exposes the quartz (C). Precipitation of kaolinite (K) likely clogs some pores, making them partly inaccessible to water. Processes of physical weathering such as freeze-melt cycling could also create new unconnected pores. At Shale Hills, chemical, physical and biological processes all can contribute to regolith formation and pore production (D). Depletion of major elements illustrates three weathering fronts at different depths (ankerite, feldspar, and clays), as seen by the negative tZr, j values (Jin et al., 2010). Water flows in the saprock and regolith dominated by advection along the bedrock/saprock interface and along fractures, but by diffusion into the grains and into the bedrock.
These observations are consistent with conversion of bedrock to saprock to regolith at SSHO due to i) transport of reactants (e.g., water, O2) into primary pores and fractures created by tectonic events and peri-glacial effects, ii) mineral-water reactions and particle loss that increase porosity and the access of water into the rock. From deep to shallow, mineral-water reactions may change from largely transport-limited where porosity was set largely by ancient tectonic activity to kinetic-limited where porosity is changing due to climate-driven processes.
[Jin, L., Rother, G., Cole, D.R., Mildner, D.F.R., Duffy, C.J. and Brantley, S.L. (2011) Characterization of deep weathering and nanoporosity development in shale – a neutron study. American Mineralogist 96:498–512.]
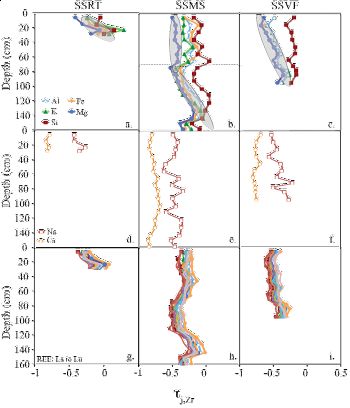
Swale Chemistry: The soil chemistry data (major and REEs) from a swale transect are characterized for comparison to similar measurements on a planar transect for the Susquehanna/Shale Hills critical zone observatory. Similar reaction fronts are observed: plagioclase dissolution is indicated by Na and Ca depletion and a negative Eu anomaly; clay dissolution followed by particle loss is accompanied by loss of Mg, K, Fe, Al and Si.
However, different from the planar transect, soils along the swale transect, especially in the topographically depressional site, do not show smooth elemental profiles. This documents both residuum soils and accumulation of colluvium sediments. The soils at the swale transect are thicker and on average wetter than those along the planar transect; however, the Ce anomaly observed in the swale soils is consistent with a generally oxic environment. Thus, preferential flowpaths are an important mechanism for water transport, preventing swale soils from water saturation.Figure 10. Depth profiles of major elements (a, SSRT; b, SSMS; c, SSVF), Ca and Na (d, SSRT; e, SSMS; f, SSVF) and REEs (g, SSRT; h, SSMS; i, SSVF) τ value as a function of depth. The uncertainties in τ calculations were estimated by error propagation and averaged at approximately 0.07 τ unit.
[Jin, L. and Brantley S.L. (2011) Soil chemistry and shale weathering on a hillslope influenced by convergent hydrologic flow regime at the Susquehanna/Shale Hills Critical Zone Observatory. Applied Geochemistry 26:S51–S56].
Deep shale weathering: Molly Holleran was a undergrad in the Geosciences Department, who worked as a field assistant at Shale Hills for the three years. She assists with lysimeter sample collection during the spring, summer and fall seasons. Molly is now working on her senior thesis based at Shale Hills, on the quantification of deep shale weathering. Research goals include evaluating the change of mineralogy with depth, and determining if changes seen can be attributed to chemical weathering or heterogeneity in the lithology. Carbonate, pyrite, feldspar, and illite/chlorite reaction fonts are being investigated, from the ridge top to the valley floor. Overall, a goal of this study is to be able to relate the process of shale weathering and associated reaction fronts with the overall water-table in the catchment.
Figure 11. Molly Holleran (undergraduate student, Geosciences) works with Danielle Andrews (graduate student, Crop and Soils Sciences) to collect water samples from lysimeters installed at SSHO.
Figure 12. Holleran assisted with water and soil collection for three years, completed a senior thesis based at SSHO, and is now pursuing a M.S. at JRB-SCM CZO.
Contacts: Susan Brantley (PI), Carmen Enid Martinez
Figure 1. A box model shows mass reservoirs of Mn (boxes) and the Mn fluxes that impact SSHO (arrows; values in μg cm-2 y-1). Emissions from anthropogenic activity have resulted in a net gain of Mn in SSHO soils (mMn, w = 47 mg cm-2). Rates of Mn uptake into trees (83 μg cm-2 y-1) currently outpace removal through chemical weathering (1 μg cm-2 y-1), indicating that vegetation may retain Mn within the catchment.
Figure 2. X-ray fluorescence spectroscopy (XRF) and X-ray absorption spectroscopy (XAS) are used to characterize the location and speciation of manganese within leaf biomass. Left: Red oak leaves examined with XRF exhibit discrete areas of high Mn, ROIs (regions of interest), as shown in red and white. Right: XAS was used to analyze Mn hot spots within foliar tissue. Mn spectra obtained from these ROIs (shown in blue) are most consistent with trivalent, organically-complexed Mn. Standards for the oxides MnO, Mn2O3, and MnO2 are also shown.
Figure 3. A contour map showing the isotopic composition of Fe in bulk soil samples (referred to as the δ56Fe value) along the transect. Contrary to expectations from experiments involving Fe-reducing bacteria and ligand-promoted dissolution, the bulk Fe signatures appear to become lighter with increased chemical weathering toward the valley floor.
Figure 4. U-series disequilibrium isotopes (238U, 234U, and 230Th) of shale rocks and soils are being analyzed at the Shale Hills catchment to derive the soil production function and estimate the residence time of regolith particles using mass balance models.
Figure 5. Based on the measured U-series activity ratios, rind formation rate is determined and increases to a factor of about 1.4 (0.23 to 0.31 mm/kyr) from a low curvature transect to a high curvature transect of the clast.
Figure 6. Mg2+, Mg*, and Mg* (Cl-normalized) concentrations in the pore water as a function of depth at the SPRT (A, D, G), SPMS (B, E, H) and SPVF (C, F, I) sites. Shaded areas highlighted the variations of Mg concentrations in soil waters from different depths. The Mg2+ concentration is that measured in the soil water; Mg* is Mg concentration in soil water after correcting for atmospheric input; and Mg* (Cl-normalized) is Mg concentration corrected for atmospheric input and evapotranspiration. Notice changes in scale between Mg2+/Mg* and Mg* (Cl-normalized).
Figure 7. Conceptual hydrological flow model on pedon (A) and hillslope (B) scales. In A, large wide black arrows indicate preferential flow paths, narrow black arrows indicate vertical flow through macropores formed for example by tree roots, and white curly arrows indicate solute exchange between low-flow zone and macropores via diffusion. In B, black arrows indicate water and Mg transport through rainfall and snowmelt input, advection along the hillslope through the preferential flow paths (high-flow domain, squares) and recharge to stream from vadose zone and groundwater. Grey arrows mean diffusion of Mg2+ from restrictive layers (low-flow domain, eclipse) to flow paths (high-flow domain). The Mg2+ concentrations in each reservoir are calculated by averaging those measured at corresponding sites/depths and used to determine the chlorite dissolution rates (see text for details). It is assumed that the annual precipitation is 1000 mm and half of it returns to the atmosphere via evapotranspiration. Thus the Mg concentration in water that penetrates to the soil doubled (=0.88 *2=1.76 mM).
Figure 8. Variations of power law Porod exponent (A), porosity (B), specific surface area (C), and ferrous and total Fe contents as a function of depth (D), for shales that have been weathered to different extents. The exponent, n, indicates the type of fractal (2 < n < 3, mass fractal; 3 < n< 4, surface fractal).
Figure 9. A schematic diagram summarizing the production and changes in connectivity of the pores as measured by neutron scattering, and the processes that are causing changes in porosity at different depths. Initially, shales are dominated by unconnected type II pores (A). With weathering, pores become more connected by loss of clays and type III pores dominate (B). With progressive weathering, more space opens up due to clay dissolution, which exposes the quartz (C). Precipitation of kaolinite (K) likely clogs some pores, making them partly inaccessible to water. Processes of physical weathering such as freeze-melt cycling could also create new unconnected pores. At Shale Hills, chemical, physical and biological processes all can contribute to regolith formation and pore production (D). Depletion of major elements illustrates three weathering fronts at different depths (ankerite, feldspar, and clays), as seen by the negative tZr, j values (Jin et al., 2010). Water flows in the saprock and regolith dominated by advection along the bedrock/saprock interface and along fractures, but by diffusion into the grains and into the bedrock.
Figure 10. Depth profiles of major elements (a, SSRT; b, SSMS; c, SSVF), Ca and Na (d, SSRT; e, SSMS; f, SSVF) and REEs (g, SSRT; h, SSMS; i, SSVF) τ value as a function of depth. The uncertainties in τ calculations were estimated by error propagation and averaged at approximately 0.07 τ unit.
Figure 11. Molly Holleran (undergraduate student, Geosciences) works with Danielle Andrews (graduate student, Crop and Soils Sciences) to collect water samples from lysimeters installed at SSHO.
Figure 12. Holleran assisted with water and soil collection for three years, completed a senior thesis based at SSHO, and is now pursuing a M.S. at JRB-SCM CZO.
-
Contacts
-
National, Eel, Luquillo, Shale Hills, INVESTIGATOR, COLLABORATOR
11 People
.(JavaScript must be enabled to view this email address)
Penn State
Aqueous Geochemistry, Geochemical Kinetics, Microbial Biogeochemistry
INVESTIGATOR, COLLABORATOR
.(JavaScript must be enabled to view this email address)
Shale weathering
.(JavaScript must be enabled to view this email address), 607/255-9354
Cornell
Biogeochemical processes
INVESTIGATOR, Visiting/Affiliate
.(JavaScript must be enabled to view this email address)
CZ development and the movement of contaminants.
INVESTIGATOR, COLLABORATOR
.(JavaScript must be enabled to view this email address)
Continental weathering
.(JavaScript must be enabled to view this email address)
Aqueous geochemistry
INVESTIGATOR
.(JavaScript must be enabled to view this email address)
multiphase flow and transport, environmental engineering, and geochemistry
GRAD STUDENT
.(JavaScript must be enabled to view this email address)
Geochemistry
INVESTIGATOR, COLLABORATOR
.(JavaScript must be enabled to view this email address)
low-temperature (bio)geochemistry, geochemistry, hydrogeology, isotope geology
GRAD STUDENT
.(JavaScript must be enabled to view this email address)
Biology - Ecology
INVESTIGATOR, Visiting/Affiliate, COLLABORATOR
.(JavaScript must be enabled to view this email address)
Isotope geochemistry
Alumni-Former
UNDERGRAD
Mathematical modeling
GRAD STUDENT
.(JavaScript must be enabled to view this email address)
Geomorphology
UNDERGRAD
.(JavaScript must be enabled to view this email address)
Hydrogeologic Modeling
GRAD STUDENT
.(JavaScript must be enabled to view this email address)
Hydrology, Biogeochemistry
COLLABORATOR, GRAD STUDENT
.(JavaScript must be enabled to view this email address)
Geochemistry
-
-
Featured Publications
2011
Soils Reveal Widespread Manganese Enrichment from Industrial Inputs. Herndon, E.M., Jin, L., and Brantley, S.L. (2011): Environmental Science & Technology 45 (1):241-247.
2010
Regolith production rates calculated with uranium-series isotopes at Susquehanna/Shale Hills Critical Zone Observatory. Ma, L., Chabaux, F., Pelt, E., Blaes, E., Jin, L., and Brantley, S.L. (2010): Earth and Planetary Science Letters vol 297, 211-225.
2011
Opening the "Black Box": Water Chemistry Reveals Hydrological Controls on Weathering in the Susquehanna Shale Hills Critical Zone Observatory . Jin, L., Andrews, D.M., Holmes, G.H., Lin, H., and Brantley, S.L. (2011): Vadose Zone Journal 10:928-942,
2011
Characterization of deep weathering and nanoporosity development in shale – A neutron study . Jin, L., Rother, G., Cole, D., Mildner, D., Duffy, C., Brantley, S.L. (2011): American Mineralogist 96:498–512.
2011
Soil chemistry and shale weathering on a hillslope influenced by convergent hydrologic flow regime at the Susquehanna/Shale Hills Critical Zone Observatory. Jin, L.,Brantley S. L. (2011): Applied Geochemistry 26:S51–S56,
Explore Further