Chen et al., 2014
Properties of Fe-organic matter associations via coprecipitation versus adsorption
Chen, C., J. Dynes, J. Wang and D.L. Sparks (2014)
Environ. Sci. Technol. 48 (23):13751–13759
-
Calhoun, Christina, INVESTIGATOR
-
Christina, INVESTIGATOR
Abstract
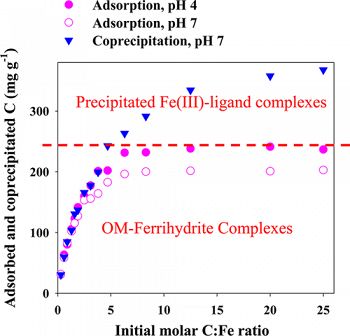
The association of organic matter (OM) with minerals is recognized as the most important stabilization mechanism for soil organic matter. This study compared the properties of Fe–OM complexes formed from adsorption (reaction of OM to postsynthesis ferrihydrite) versus coprecipitation (formation of Fe solids in the presence of OM).
Coprecipitates and adsorption complexes were synthesized using dissolved organic matter (DOM) extracts from a forest little layer at varying molar C/Fe ratios of 0.3–25.0. Sample properties were studied by N2 gas adsorption, XRD, FTIR, Fe EXAFS, and STXM-NEXAFS techniques. Coprecipitation resulted in much higher maximum C contents (∼130 mg g–1 C difference) in the solid products than adsorption, which may be related to the formation of precipitated insoluble Fe(III)–organic complexes at high C/Fe ratios in the coprecipitates as revealed by Fe EXAFS analysis. Coprecipitation led to a complete blockage of mineral surface sites and pores with ≥177 mg g–1 C and molar C/Fe ratios ≥2.8 in the solid products. FTIR and STXM-NEXAFS showed that the coprecipitated OM was similar in composition to the adsorbed OM. An enrichment of aromatic C was observed at low C/Fe ratios. Association of carboxyl functional groups with Fe was shown with FTIR and STXM-NEXAFS analysis. STXM-NEXAFS analysis showed a continuous C distribution on minerals. Desorption of the coprecipitated OM was less than that of the adsorbed OM at comparable C/Fe ratios.
These results are helpful to understand C and Fe cycling in the natural environments with periodically fluctuating redox conditions, where coprecipitation can occur.
Citation
Chen, C., J. Dynes, J. Wang and D.L. Sparks (2014): Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ. Sci. Technol. 48 (23):13751–13759. DOI: 10.1021/es503669u
Explore Further