CZ-tope Research Group
CZ-Tope is an innovative international effort to promote isotopic measurements on identical critical zone samples. Right now, this work is highly useful because many “nontraditional” isotopic systems are now being developed and have not yet been tested thoroughly in the field. For CZ-Tope so far, these isotopes have been measured or proposed at SSHCZO to be measured on identical samples: 2H, 18O, B, 10Be , C, Ca, Cd, Cu, Ge, Li, 56Fe, Mg, S, Si, and U series.
This group is tagged with:
-
Feedbacks among weathering, erosion, and other biogeochemical processes make quantification and modeling of the Critical Zone (CZ) difficult. Mass-dependent fractionation of many elements can be used to fingerprint CZ material fluxes. Now researchers can use multiple isotopes (O, H, C, S, Ca, Mg, Sr, Li, B, Be, etc.) in the same setting to elucidate complex systems. In addition, metal stable isotopes (e.g. Cu, Zn, Cd, Hg, and Pb) can fingerprint anthropogenic influences. Still, there is a need to develop better approaches toward using multiple isotopes to compare biogeochemical interactions in environments of differing lithology and environmental characteristics.
Boron (B) Jérôme Gaillardet and Johanna Noireaux (Institut de Physique du Globe de Paris, France)
Boron has two isotopes, 10B and 11B that are naturally present on Earth. The average ratio of 11B/10B is about 4, however, subtle differences exist between water and rocks. Classically, we express the boron isotopes by the deviation of an international standard, in ‰. Previous work by Lemarchand et al. (2001) exemplified that dissolved boron in rivers is 10‰ enriched in 11B compared to the upper continental crust. Dissolved boron is therefore “ heavy” compared to crustal boron. The origin of this isotope discrimination is due to the preferential incorporation of 10B in the secondary phases formed during weathering and to the preferential uptake of boron by the vegetation. Clays and plants are “light” for boron. The project aims at analyzing boron isotopes in the different compartments of the SSHCZO, to explore the isotopic fractionation generated by water rock interactions within the watershed and to constrain the links between chemical weathering of shales and vegetation activity.
Boron will be measured by MC-ICPMS in Paris, after chemical extraction from geological or biological matrix.
Calcium (Ca) Matthew Fantle (Pennsylvania State University, USA) and Grit Steinhoefel
Lithium (Li) Maureen Fieneman (Pennsylvania State University, USA)
Carbon (C) Lixin Jin (University of Texas at El Paso, USA)
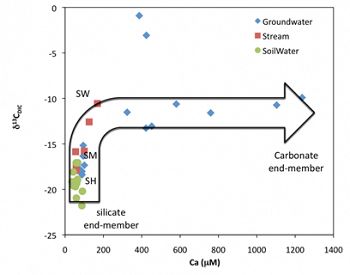
C concentrations and isotopic compositions (δ13C) of different reservoirs are studied in the Susquehanna Shale Hills Critical Zone Observatory, including organic matter in the soils and Rose Hill bedrock, carbonate minerals, CO2 in the soil gas, and dissolved inorganic carbon (DIC) and DOC in the natural waters (soil water, ground water and stream). The goals are: 1) to evaluate the degradation of organic matter in controlling seasonal variations of soil CO2 concentrations; 2) to determine the relative contribution of silicate and carbonate dissolution to overall stream DIC; 3) to identify the sources of stream DIC: soil CO2, atmospheric CO2 and/or carbonate C; and 4) to quantify the CO2 budget over the whole watershed by determining the mineral weathering rates and organic matter decomposition rates. This case study will help understand C fractionation in the surface and subsurface and develop C isotopes as an environmental tracer. More importantly, it will allow us to evaluate the roles of shale in global CO2 cycles.
The evolution of natural waters based on elemental chemistry and carbon isotopes: shallow soil water is characterized by low Ca concentrations from silicate weathering and depleted δ13CDIC values (controlled by equilibrium with soil CO2) while groundwaters are dominated by carbonate dissolution with much higher Ca concentrations and constant δ13CDIC values (controlled by mixing of ankerite and CO2 carbon).
Magnesium (Mg) Lin Ma (University of Texas at El Paso, USA), F. Zhen
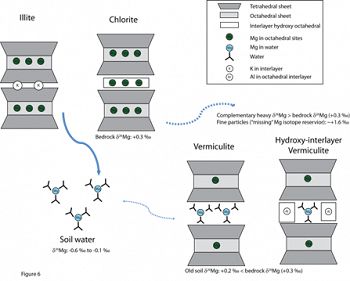
The behavior of Mg ions and their transport during biogeochemical cycling at Earth’s surface is closely linked to long-term changes in Earth’s climate. Mg stable isotopic ratios have great potential as a natural tracer to elucidate weathering processes and biogeochemical pathways in surficial environments. Here, we investigated the Mg isotope fractionation during shale weathering under a temperate climate in northeastern USA. Significant fractionation of Mg isotopes during shale weathering is clearly observed at Shale Hills: δ26Mg values of stream and soil pore waters are about ~0.5 ‰ to 1 ‰ lighter than the bedrock. Most soil samples show distinctly lighter Mg isotope compositions compared to the bedrock. We suggest that the accumulation of light Mg isotopes in soils at Shale Hills is due to i) mobilization of isotopically light Mg solute during clay dissolution and re-precipitation as vermiculite or HIV; and ii) loss of isotopically heavy particulate Mg. Our study provides the first field evidence that changes in clay mineralogy affect the Mg isotope compositions of residual bulk soils. This example also demonstrates a new and important mechanism – loss of isotopically distinct fine particles from clay-rich systems -- that could drive the Mg isotope compositions of weathering residuals in an opposite direction as normally expected from silicate weathering, i.e. to lighter Mg values instead of heavier values.
A conceptual model showing mineral structures and Mg mobility and exchange for primary Mg-rich minerals illite and chlorite, and secondary Mg-rich mineral vermiculite. It is suggested that vermiculite can trap dissolve Mg2+ ions with light Mg isotopic compositions from circulating soil waters in its interlayer structure, leading to lower δ26Mg values in older soils with high contents of vermiculite and hydroxyl-interlayer vermiculite. The inferred “missing” heavy Mg isotope reservoir is related to the loss of particles at Shale Hills.
Source: Ma et al. (in review), Mg isotope fractionation during shale weathering in the Shale Hills Critical Zone Observatory: Accumulation of light Mg isotopes in soils by clay mineral transformation, Chemical Geology.
Lead (Pb) and Cadmium (Cd) Lin Ma (University of Texas at El Paso, USA)
Human activities have significantly increased the metal emission to the atmosphere, and these metals are subsequently deposited back to Earth’s surface, disrupting their natural biogeochemical cycles. Elevated concentrations of heavy metals have been documented in water and at the land surface on a global scale. The Shale Hills CZO is located in a small temperate watershed in Pennsylvania. This watershed experienced relatively minimal human disturbances in the past. However, detailed soil characterization (Herndon et al., 2011) has revealed the presence of unusually high levels of Mn, Pb and Cd in surface soils due to atmospheric deposition. The metal contamination can be attributed to several emission sources including: 1) iron production in the 19th century in Pennsylvania; 2) steel plants and coal-burning power plants; and 3) contributions from gasoline. Contribution from iron production was highlighted as particularly important. That interesting study invoked more questions regarding the historical metal loadings at Shale Hills and northeastern USA which we investigate in this study. Specifically, we sought to understand whether the iron production also led to enrichment of metals such as Pb and Cd in surface soils and, if so, whether Pb and Cd isotopes could be used to determine the local and/or regional sources of the metal loadings, including whether the metal signatures are derived from the early industrial revolution period or modern industrial activities.
Silicon (Si) Friedhelm von Blanckenburg (Department 3 “Geodynamics”, Postdam)
Strontium (Sr), Germanium/Silicon (Ge/Si) Louis Derry (Cornell University, USA)
210Pb and 137Cs Diana Karwan (University of Minnesota, USA)
Oxygen (δ18O) and Deturium (δ2H) Christopher Duffy (The Pennsylvania State University, USA) and Evan Thomas (The Pennsylvania State University, USA)
Beryllium (10Be) Paul Bierman (The University of Vermont, USA) and Nicole West (The Pennsylvania State University, USA)
Iron (δ56Fe) Tiffany Yesavage (The Pennsylvania State University, USA) and Susan Brantley (The Pennsylvania State University, USA)
Copper (Cu) Ryan Mathur (Juniata College, USA)
The evolution of natural waters based on elemental chemistry and carbon isotopes: shallow soil water is characterized by low Ca concentrations from silicate weathering and depleted δ13CDIC values (controlled by equilibrium with soil CO2) while groundwaters are dominated by carbonate dissolution with much higher Ca concentrations and constant δ13CDIC values (controlled by mixing of ankerite and CO2 carbon).
A conceptual model showing mineral structures and Mg mobility and exchange for primary Mg-rich minerals illite and chlorite, and secondary Mg-rich mineral vermiculite. It is suggested that vermiculite can trap dissolve Mg2+ ions with light Mg isotopic compositions from circulating soil waters in its interlayer structure, leading to lower δ26Mg values in older soils with high contents of vermiculite and hydroxyl-interlayer vermiculite. The inferred “missing” heavy Mg isotope reservoir is related to the loss of particles at Shale Hills.
-
Contacts
-
National, Eel, Luquillo, Shale Hills, INVESTIGATOR, COLLABORATOR
5 People
.(JavaScript must be enabled to view this email address)
Penn State
Aqueous Geochemistry, Geochemical Kinetics, Microbial Biogeochemistry
INVESTIGATOR
.(JavaScript must be enabled to view this email address)
Penn State
Stochastic and numerical modeling of groundwater flow and solute transport, modeling large-scale hydrologic systems.
INVESTIGATOR, COLLABORATOR
.(JavaScript must be enabled to view this email address)
Continental weathering
INVESTIGATOR, COLLABORATOR
.(JavaScript must be enabled to view this email address)
Isotope geochemistry
Cross-CZO INVESTIGATOR, Visiting/Affiliate, COLLABORATOR
.(JavaScript must be enabled to view this email address)
KU
Hydrogeochemistry
-
Explore Further